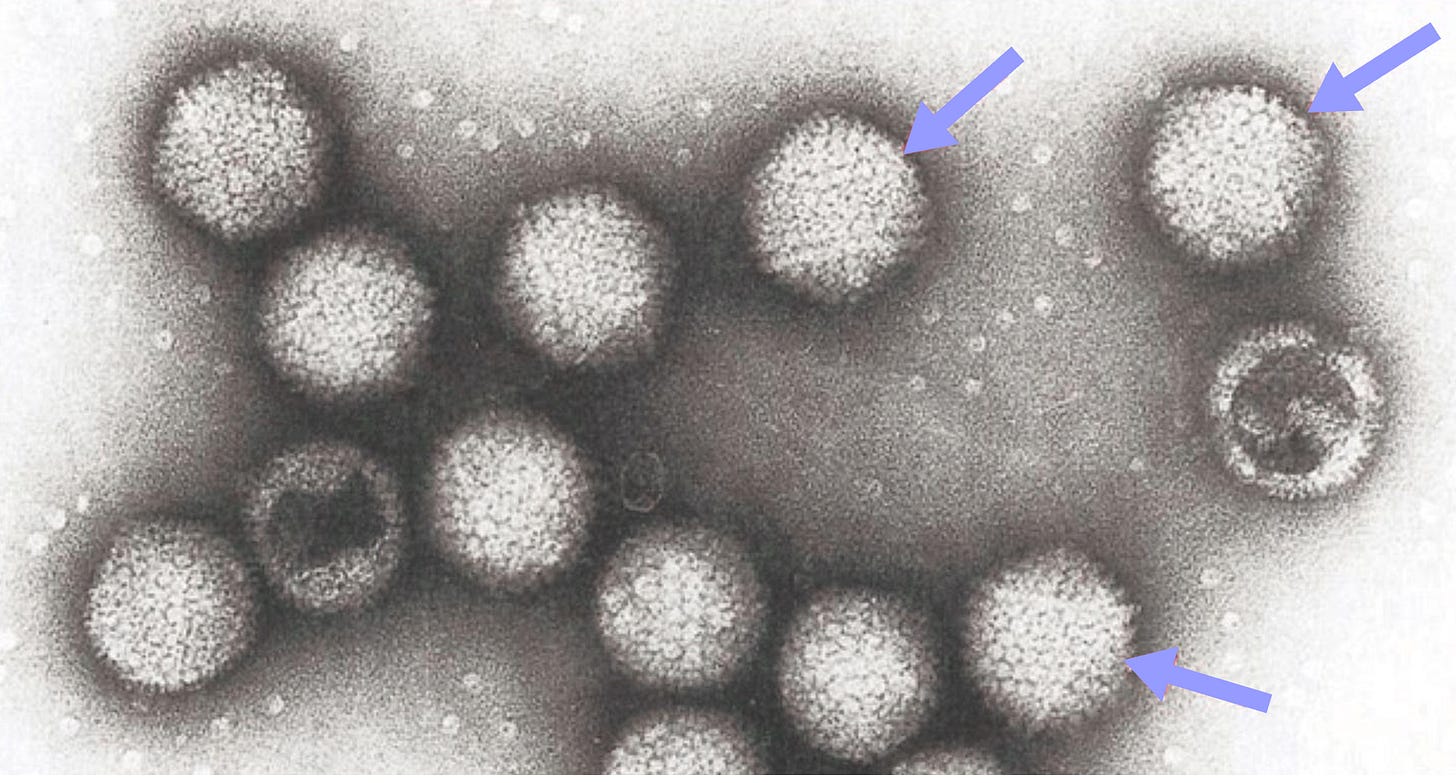
Why Haven’t Adenoviruses Caught up to AAVs for Gene Therapy?
The vast adenoviral cargo space could reward the gene therapy field, but it comes at the cost of added "manufacturing taxes"
In the age of gene therapy, the decisive battle will no longer be fought in target discovery, but in ferrying therapeutic nucleic acids, efficiently and specifically, to the cells that require them.
Our capacity to design therapeutic cassettes already outruns our means of efficient and targeted delivery. This asymmetry is laid bare by nadofaragene firadenovec (Adstiladrin), still the lone FDA-approved non-oncolytic adenoviral therapy nearly four decades after adenoviruses first entered the conversation.
Instilled directly into the bladder, Adstiladrin expresses its cargo, a gene encoding interferon-alpha, turning the urothelium into a transient factory of cytokine and, for many patients, a last line of defense. The choice of adenovirus here was deliberate: generous packaging capacity, efficient transduction of the urothelium, and, very importantly, a local delivery route that dodges the systemic antibodies that would neutralize it.
Yet this local triumph highlights the grand impasse of our field. Safe, precise, efficient delivery. Three criteria—tissue specificity, adequate and optimally timed therapeutic expression, patient safety—compose a deceptively simple rubric that still eludes universal solutions. Public fascination often lingers on gene editing breakthroughs or therapeutic applications against targets for interesting traits or diseases, while the persistent challenges, bottlenecks and inefficiencies in vector engineering draw less attention.
Reality is, we have become quite adept at designing therapeutic cargos, and waiting for delivery systems worthy of their contents.
Adeno-associated virus (AAV) reigns today by virtue of low immunogenicity, lower pre-existing seroprevalence in the general population, and capacity for long-lasting transgene expression. Its crown, though, is circumscribed by a rigid ~4.7kb carrying capacity. Anything bulkier (multiplexed gene circuits, complete CRISPR/Cas9-based editing systems, large genes) cannot be accommodated without modifications or sophisticated engineering (some examples here), if at all. Adenovirus, by contrast, can accommodate up to ~36kb of cargo, over sevenfold that of AAV. Yet its applications in systemic gene therapy have stalled, encumbered by high immunogenicity, its sequestration in the liver after administration, and, less visibly, a crushing difficulty in its process development, which is highly inefficient.
We have witnessed extensive research efforts to engineer AAV capsids for altered tropism. Comparable attempts for adenovirus feel conspicuously absent. Given their substantial advantage in cargo capacity, wouldn't enhancing adenoviral vectors be a highly valuable endeavor? Why has progress in adenoviral vector engineering appeared to stagnate over the past few decades compared to AAVs?
This essay traces those fault lines. Drawing on years spent working with adenoviruses during a viral vector-heavy PhD and work within and alongside a viral vector CDMO, I will peel back the shorthand of “higher immunogenicity” to expose the safety and manufacturing realities that have quietly shaped the viral vector landscape.
Gelsinger’s death was only part of the story
Viral vectors have been used to deliver genetic cargo in human clinical trials since the 90s.
Initially, the adenovirus was among the most favoured choices. It was fairly well understood and characterised back then, leading to its preferred use relative to other viral systems like AAV, which was then a nascent technology with its first gene therapy trials emerging later in that decade.
That centripetal pull did not last. On 13 September 1999, eighteen-year-old Jesse Gelsinger received 3.8x10¹³ particles of an adenovirus 5 vector carrying OTC cDNA, which was reported as “the highest [dose] so far with an adenovirus” that any human had ever seen in a single infusion. Within hours he spiralled into a cytokine storm and multi-organ failure, dying four days later. The shockwave was immediate. The FDA halted every gene-therapy protocol at Penn’s Institute for Human Gene Therapy in January 2000, and the field slipped into its long, mis-named “dark age.” (Dark, perhaps, in funding, not in insight.)
Yet the common retelling, that one tragic death single-handedly derailed adenoviral medicine, misses a quieter, more pervasive drag, the engineering surcharge built into the biology of the virus itself. Even before 1999, investigators understood that intravenous adenovirus provokes a strong inflammatory response, and they had already carved away its early genes in an attempt to tame that response. “Third-generation,” or so-called “gutless”, adenoviral vectors, entirely empty of coding sequence, were prototyped by 1998. Each revision, however, exacted a manufacturing tariff. Every gene excision demanded new helper plasmids, new producer lines, new purification logic. Immunogenicity could be blunted, but only by leaning harder on process development already groaning under the ~36kb size of the adenoviral genome.
Meanwhile, the AAV, significantly smaller and capped at a paltry ~4.7kb, began a quieter ascent. Subretinal injections for Leber congenital amaurosis (2008) and hepatotropic fixes for hemophilia (2010-2012) produced safe, durable expression1. Capital followed data, and the centre of gravity in vectorology shifted almost imperceptibly. Between 2004 and 2023, adenovirus crept from 240 to 574 registered clinical trials, a respectable 2.4-fold rise. AAV, starting from nineteen, detonated to 366, a nineteen-fold leap that left it commanding roughly half of all new adenovirus-or-AAV studies launched since 20042.
Had Gelsinger lived, this divergence would likely have persisted. The constraints are biological and logistic, not merely historical. Adenovirus tempts with large cargo capacity suited to long gene delivery, but it will just as reliably challenge manufacturing both at pre-clinical and clinical scales. AAV, by contrast, forces minimalism in transgene design but rewards obedience with tractable upstream and downstream workflows.
Gene therapy did not abandon adenovirus after 1999. It simply recalibrated its expectations. We still recruit the vector where its packaging capacity justifies the process complexity, and to harness its inherent immunogenicity: oncolytic virotherapies, and vaccine development platforms. But for systemic, chronic delivery, the field found it easier to miniaturize the cassette than to domesticate the giant.
The adenoviral manufacturing tax
Adenovirus genomes are compact but busy. In human serotype 5, the workhorse of most adenoviral vector programs, about 36kb of linear, double-stranded DNA encodes three timed sets of genes: early (E1A/B, E2, E3, E4), intermediate (IX, IVa2) and late (L1-L5) (x). In brief summary, E1A turns on the rest, E2 supplies the replication enzymes, E3 modulates host immunity, and the late genes build the capsid and finish assembly.
Vector design starts by deleting what you can live without. First-generation constructs drop E1 (essential for replication) and usually E3 (dispensable). That frees ~8.2 kb for a therapeutic cassette, roughly ~1.75x that of typical AAV vectors. Due to deletion of the required replication program orchestrator E1, expression of late genes should not occur, in theory. In reality, however, “leaky” (low-level) expression of remaining viral genes, particularly late genes, can still occur, likely due to the abundance of host transcription factors in host cells. This residual viral gene expression enhances immunity to first-generation vectors, initially provoked by the capsid proteins.
Production of these E1-deleted vectors requires specialized packaging cell lines that stably express the E1 proteins in trans (e.g. the HEK239T derivative Ad239T, and Janssen’s PER.C6) to complement the E1 deletion. This allows the vector DNA, typically introduced as a plasmid containing the transgene within the E1-deleted adenoviral backbone, to replicate and be packaged into virions. Vectors with deletions in E1 and E3 (or E1 alone) constitute the “first-generation” of adenoviral vectors, a design still employed, for example, in Adstiladrin.
Given that immunogenicity is the major hurdle of field-wide adoption of adenoviral vectors, deleting additional replication units to remove the adaptive arm of the immune response has been explored as a strategy for generating subsequent generations of adenoviral vectors.
Second-generation vectors deleted extra replication units (E2 or E4) to cut immunogenicity further. Results were inconsistent. Some experimental models showed longer expression, others saw no change. Manufacturing, however, clearly suffered. Every deleted gene had to be provided in trans, forcing increasingly complex producer cell lines and lower yields. The cost-benefit balance pushed most programs back to first-generation simplicity.
Third-generation, “helper-dependent” or “gutless,” vectors take a different approach. They remove nearly everything except the inverted terminal repeats and packaging signal. A separate helper virus supplies all replication functions. This largely avoids adaptive immune activation and opens the full ~36kb cargo hold. To put this into context, 36kb is massive, enough to deliver entire CRISPR-Cas9 systems, including the Cas9 and guide RNA, without Cas9 modifications, something beyond the capabilities of a single AAV vector. In vivo studies dating since the 90s showed promising results in increasing the duration of transgene expression through a gutless adenoviral vector compared to previous generations, which was sustained for up to a year in a baboon model. They still induce some innate immune activity, but strikingly, it has been demonstrated that they could drive year-long gene expression in the brains of mice even when the animals were already pre-immunised against adenovirus.
The helper virus used for gutless adenoviral vector production is similar in composition to first-generation adenoviral vectors. It is typically modified with loxP sites bracketing its packaging signal, so Cre recombinase (typically expressed by HEK293 or HEK293-like cell lines) can cut that signal out. As a result, the helper virus genome remains un-packageable but still able to replicate, therefore trans-complimenting the replication of the gutless adenoviral genome. In practice, some helper virus slips through, and that residual, fully immunogenic adenovirus is the main manufacturing tax on third-generation platforms. Recent exploratory research work in vector engineering led to the development of novel methods for gutless vector production, including the use of a helper plasmid instead of a helper virus, as well as self-inactivating helper viruses whose packaging signal auto-disrupts after one round.
With gutless delivering both the space and the immunology benefits, as well as its continuing capacity for improvement from a manufacturing standpoint, 3rd generation has grown into an interesting candidate in the current vector development landscape.
Why does barely anyone build adenoviral libraries?
The adenoviral capsid is an icosahedral structure composed of hexon, penton-base, and fiber proteins. Several minor capsid proteins (IIIa, VI, VIII, IX) also contribute. Which cells get transduced by the adenoviral capsid depends on its taxonomy, with different species and serotypes preferring different cell surface receptors for entry into the host. Species C adenoviruses, which include the adenovirus 5, use the coxsackie-adenovirus receptor (CAR), many species B use CD46 or desmoglein-2, while some other strains bind sialic acid. Because these receptors are patchily expressed, they dictate tropism.
Adenovirus 5 chiefly targets the airway epithelium in the respiratory tract, yet the instant it meets blood it is hijacked. Coagulation factor X binds the capsid and complement proteins tag it for clearing. Factor X in particular ferries large swaths of the dose onto heparan-sulfate moorings on hepatocytes, so the liver becomes the unintended sink. This hepatic sequestration, also mirrored in AAV, underlies the dose-limiting liver toxicities that have stalked both platforms and keeps “de-target the liver” an important vector engineering issue that needs to be addressed.
Huge adenoviral cargo capacity is a headline, but tropism and liver sequestration remain gatekeepers for efficient targeted delivery in vivo.
Capsid engineering remains the most direct way to steer cargo to discrete cellular niches while preventing off-target transgene expression in non-target cells. For decades in the adenovirus field, our response for tackling tropism-related problems has been rational design. Although great in theory, rational design is not applicable to target cell types to which the optimal vector modifications are not known in advance. Fiber protein, protein IX, and hexon modifications have all nudged adenoviral vectors toward new cell types, but each success is artisanal and painfully non-scalable.
Directed evolution, which mimics natural cycles of random mutation and positive selection, can remove many current bottlenecks in vector engineering. By unleashing libraries of capsid variants—each harboring random peptide sequences inserted at specific positions in viral proteins, recombined genomic cassettes from various parental viruses to create chimeric vectors, or random point-mutations—we let selection adjudicate which virions thrive in the crucible of a chosen tissue.
Directed evolution has shown significant promise for AAVs. AAV2/7m8 emerged from a peptide-display library and now headlines multiple ocular gene-therapy trials. Adenovirus, by contrast, has produced very few novel capsids though directed evolution approaches, with just one having advanced into the clinic: ColoAd1 (Enadenotucirev). ColoAd1 was the first chimeric adenoviral capsid generated via directed evolution in 2008. It was developed by recombining the species B adenoviruses 3 and 11, and selecting replication-competent variants by serial passage of the generated library in colon adenocarcinoma cell lines.
Paradoxically, the same features that make adenovirus attractive as a vector impose further manufacturing taxes. Scaling a viral genome variant library for downstream applications including directed evolution studies has two requirements: first, rapidly generating a large set of distinct variants, and second, producing those variants economically at volumes suitable for screening. Adenovirus challenges both goals. Its ~36 kb genome demands more complex cloning, each construct typically needs to be amplified in cells multiple times to achieve adequate yields, and the virus’s strong cytopathic effect limits culture density and yield. Together, these factors make adenoviral library production slower, costlier, and more space-intensive than the streamlined AAV workflow.
For recombinant adeno-associated virus (rAAV) those goals are reached in days, whereas for E1-deleted adenovirus they stretch into weeks. Current library-building workflows typically rely on bacterial artificial chromosome (BAC) systems, mainly because bacteria are convenient. Convenient, until we discover that 1ug of a 36kb recombinant adenoviral genome (~3x10¹⁰ genome copies) yield a mere ~50 infectious viral particles. In other words, just 1 in 6x10⁸ genomes successfully produces an infectious virus. This inefficiency stems from the fact that DNA propagated in bacteria lacks the virus encoded terminal proteins that normally cap adenoviral genomes and facilitate its import into the nucleus of the producer cells, where its replication occurs. Without them, terminal protein deficient genomes are 100- to 1000-fold less competent at producing infectious particles than their naturally terminal protein-capped counterparts.
According to an estimate in a study using random peptide insertion into the HI-loop region of the adenoviral fiber protein, ~10⁴ functional variants were generated per well. Extrapolating from that number, and allowing for the 90% of the peptide insertions that can hinder virion production, approaching the theoretical 10⁹-member ceiling of possible genome variants would require on the order of 1,000 six-well plates.
Consequently, rescuing virus stocks requires multiple rounds of amplification in ever-larger cell cultures, stretching the timeline from recombinant DNA to high-titre material to as long as two months. This constricts our capacity to generate large, diverse adenoviral genome libraries, a variety which is indispensable for isolating desirable capsid mutants. We cannot screen what we cannot build.
Several (though not enough) strategies have emerged, including ADEVO and FastAd, where various modifications applied to traditional adenoviral library preparation protocols resulted in faster workflows and an increase in the number of variants generated. However, these improvements do not include practical attempts at scaling them for a large adenoviral directed evolution effort to identify novel variants with altered tropism.
Could that bottleneck finally begin to crack? Various steps in the adenoviral library preparation workflow could adopt automation systems that have been thoroughly developed for AAV library preparation elsewhere. If the field can reduce the manufacturing tax for clinically viable applications while preserving adenovirus’s natural payload advantage, we reclaim an entire therapeutic design space for long cargo delivery that AAV simply cannot straight-forwardly reach.
Third-generation adenoviral vectors remove most of the immunogenic parts and open the entire ~36kb hold, but the decisive tests lie ahead. Can the newer helper-virus-free systems hit commercial-scale titers that are clinical-grade? Will we succeed in making adenoviral vector library screens routine? Could capsid engineering do the same for adenoviral vectors like it did for AAVs, provided we decide it’s worth the effort? The answers will determine whether “long-gene delivery” becomes an option within the viral vector alley or remains a promising capability looking for a tractable platform elsewhere.
The seminal AAV8-FIX study showed multi-year factor IX activity (2–11 % of normal) and a favourable safety profile. Later cohorts required transient steroids for liver-enzyme flares.
Those numbers were assembled from the multi-decade-long collective effort by multiple scientists of reporting gene therapy clinical trial statistics (2004 report, 2007 report, 2012 report, 2023 report.)